How do you measure the edge of a body centered cubic. Body-centered cubic BCC is the name given to a type of atom arrangement found in nature.

Cubic Lattice From Wolfram Mathworld Cell Forms Unit Cell Lattice
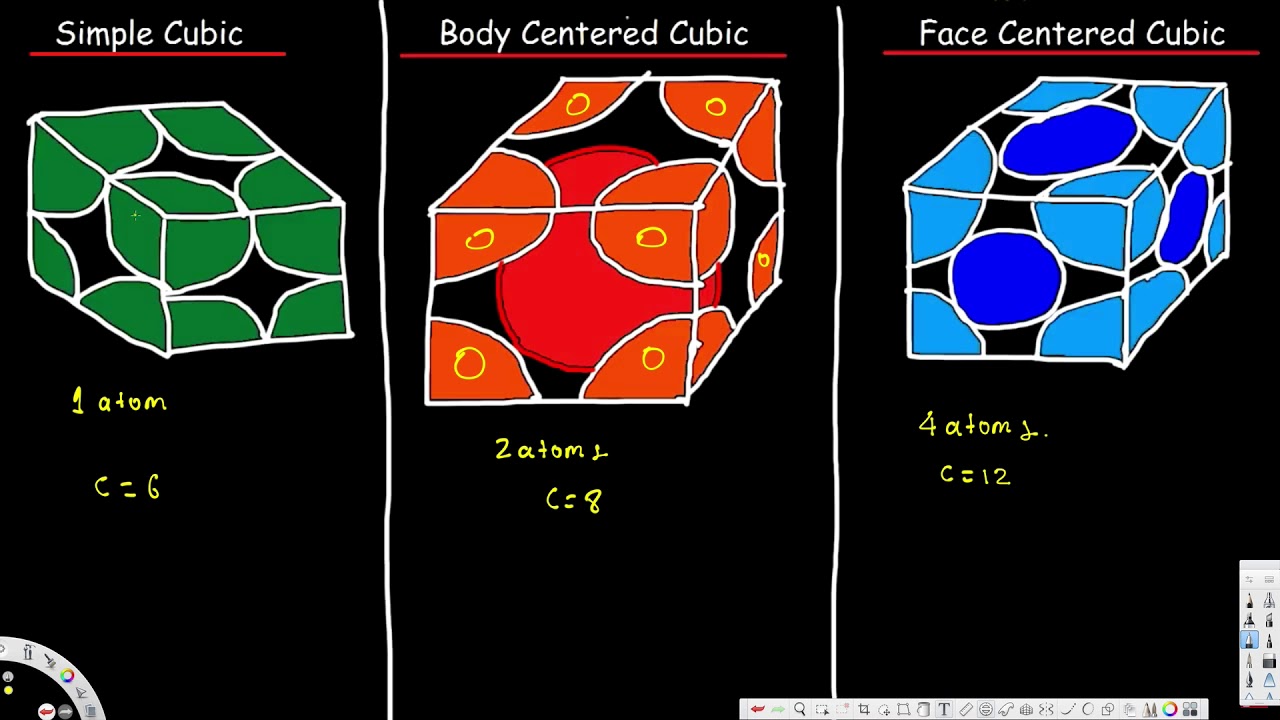
Therefore the total number of atoms in one unit cell is 8 18 1 atom.

. Find the atomic radius of gold. Vanadium has a body-centered cubic structure. Thus the unit cell of each bcc.
The body-centered tetragonal unit cell can be imagined as a cube that is slightly taller or shorter in one direction with an atom on each corner and in the very center. In the body-centered cubic lattice a total 4 radius one complete atom and two half atoms participate at the body diagonal. There is one atom or ion in the center of the unit cell in addition to the corner atoms or ions.
425 52 votes. A body-centered cubic unit cell structure is composed of atoms organized in a cube with one atom in each corner and one atom in the center. 1 simple cubic 2 face-centered cubic and 3body.
A body-centered cubic unit cell structure consists of atoms. What is body-centered cubic unit cell. In each cubic unit cell there are 8 atoms at the corners.
In the body-centered cubic lattice a total 4 radius one complete atom and two half atoms participate at the body diagonal. This is called a. How do you measure the edge of a body centered cubic.
Tantalum forms a body-centered cubic unit cell with a3302 mathrmpm. 4 Atomic packing factor in body centred cubic unit cell is 68 percent. A body-centered cubic unit cell structure consists of atoms arranged in a cube where each corner of the cube shares an atom and with one atom positioned at the.
A 533 gcm3 b 429 gcm3 c 571 gcm3 d 052. Eight other unit cells share the atom at the. There are also atoms or ions.
Body-centred Cubic Unit Cell BCC source. Calculate the crystallographic radius of a tantalum atomWatch the full video athtt. As one example the cubic crystal system is composed of three different types of unit cells.
425 59 votes. If the atomic radius of vanadium is 134 pm calculate the density of solid vanadium. It highlights the key differences between the sim.
The body-centered cubic bcc lattice Figure 14b can be obtained by adding a second lattice point at the center of each cubic cell of a simple cubic lattice. 5 Relationship between cube edge length. Naturally occurring gold crystallises in face centred cubic structure and has a density of 193gcm.
Au 197 gmol A. Some metals crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and an atom in the center as shown in Figure 2. Atomic packing factor in face-centred cubic unit cell has 12.
Unit cells occur in many different varieties. This chemistry video tutorial provides a basic introduction into unit cell and crystal lattice structures.
Unit Cell Simple Cubic Body Centered Cubic Face Centered Cubic Cryst Unit Cell Crystal Lattice Structure Nomenclature Chemistry

Unit Cell Chemistry Atomic Radius Density Edge Length Calculations Unit Cell The Unit Atom

Unit Cell Body Centered Cubic Crystal Lattice Structures Physical E Crystal Lattice Structure Unit Cell Nomenclature Chemistry

Comparison Of Space Filling In Different Cubic Structures Unit Cell Material Science Physical Chemistry
0 Comments